State Hygienic Laboratory
COVID-19 Testing
Information and Updates for
Healthcare Providers and Clinical Laboratories
Test Results
Tests ordered by a healthcare provider or clinical laboratory are posted to the SHL Web Portal upon completion, generally 24-72 hours from the time the specimen arrives at the lab.
Order a Test
Long Term Care Facilities and Clinical Labs must submit COVID-19 samples using the Electronic Test Request Form in the SHL Web Portal to ensure a timely result.
Hours
Sample Receiving Window Hours
2490 Crosspark Road, Coralville, IA 52241
Sat: 9:00 AM to Noon
Sun: CLOSED
Lab Advisory: CMS Guidance for the Use of Expired SARS-CoV-2 Tests
To address the concern about SARS-CoV-2 testing reagents and swab supply shortages during the COVID-19 public health emergency, the Centers for Medicare & Medicaid Services (CMS) will allow laboratories and testing sites to use expired SARS-CoV-2 test kits, reagents, and swabs—unless doing so deviates from the test manufacturer’s authorized instructions for use.
When using expired reagents, it is important to run controls.
Additional Details: CMS Guidance for the Use of Expired SARS-CoV-2 Tests (cdc.gov)
Updates
Disposal of Expired VTM and Swabs
SHL to End Weekend COVID Testing
State Ending COVID-19 Mandatory Reporting
FDA Update to COVID-19 Antigen Test EUAs
CMS Update for the Use of SARS-CoV-2 Tests on Asymptomatic Individuals
Test Iowa At-Home
Test Iowa At-Home
Test Iowa At-Home is a partnership between the Iowa Department of Public Health and the State Hygienic Lab. Our goal is to ensure all Iowans have access to free COVID-19 testing.
For information or to order a kit, visit https://www.testiowa.com/en.
Test Kit Storage & Transport in Winter Temperatures
- Storage of test kits: The State Hygienic Laboratory has validated the storage of the test kits before saliva collection and found that there is no impact on freezing the collection device before sample collection on the test performance. If the blue stabilization solution is frozen, please allow approximately 10 minutes at room temperature for it to thaw before collecting a saliva sample.
- Transport of test kits: Per the EUA for the test kit, a collected saliva specimen can be transported over the wide temperature range experienced in Iowa before testing at SHL. Therefore, if the sample in the blue fluid with saliva is frozen, there is not a problem.
Diagnostic PCR Testing
Testing Criteria
Testing Criteria
On February 9, 2021, the Iowa Department of Public Health eliminated PCR testing criteria for SARS-CoV-2 at the State Hygienic Laboratory. SHL will accept any PCR detection test for COVID-19 infection ordered by a healthcare provider with no restrictions.
Test Performance Characteristics
The State Hygienic Laboratory has validated multiple Molecular Diagnostic Tests for SARS-CoV-2 that previously received emergency use authorization from the FDA. Performance characteristics for each assay are contained within the Instructions for Use as issued by the manufacturer within the links below. There are not comparison standards for these tests. Test performance characteristics are based on specimen collection per the manufacturers recommendation.
If you suspect a patient is infected with COVID-19 and the test is negative, you may repeat the test.
The State Hygienic Lab currently performs the following Molecular Diagnostic Tests for SARS-CoV-2 (EUA):
- ThermoFisher Scientific TaqPath™ COVID-19 Real-Time PCR Combo Kit
(Updated EUA issued 02/23/2021)
Performance characteristics: https://www.fda.gov/media/136112/download
- Hologic Inc Panther Fusion SARS-CoV-2 Assay
(Updated EUA issued 07/23/2021)
Performance characteristics: https://www.fda.gov/media/136156/download
Cycle Threshold (Ct) Values in PCR Tests
The State Hygienic Laboratory (SHL) understands the public’s interest in ensuring the accuracy and reliability of the testing methods it uses to detect the presence of the virus that causes COVID-19 in swab samples sent to it from across Iowa. SHL shares that concern and is committed to providing timely results that are as accurate as possible with the current equipment, well trained staff, and best science.
The SHL adheres to rigorous, nationally recognized standards governing the handling of specimens, the validation of the equipment used to process tests, daily quality control, and other quality management protocols for analyzing and reporting out results. That includes strictly following the testing procedures spelled out by the manufacturers of the lab equipment used by SHL.
Cycle Threshold (Ct) values are used in polymerase chain reaction (PCR) tests. However, molecular biology is a complex science and attempting to link specific Ct values to particular results can be misleading. The Ct value above or below the threshold does not alone directly correlate to a positive or negative result; it’s only one portion of result analysis. SHL, for instance, also looks at the slope of the PCR curve depicted in the results to indicate how the amplification of the DNA is occurring.
SHL does not track or aggregate Ct values as part of its reporting process because it is not a recommended practice nor considered useful by national testing standards. Notably, the Association of Public Health Laboratories, Centers for Disease Control, and the College of American Pathologists have all clearly stated that Ct values should not be reported because of variations in sample collection and testing.
SHL reports patient results as positive, negative, or inconclusive. (In the latter case, the recommendation is to collect another sample from the patient.)
You may find these resources helpful for better understanding the role of Ct values in COVID-19 testing:
- Interpreting Results of Diagnostic Tests, from Centers for Disease Control’s Frequently Asked Questions about Coronavirus (COVID-19) for Laboratories (Serology)
- Ct Values: What They Are and How They Can be Used, by the Association of Public Health Laboratories (November 9, 2020)
- College of American Pathologists (CAP) Microbiology Committee Perspective: Caution Must Be Used in Interpreting the Cycle Threshold (Ct) Value, by the Infectious Disease Society of America
Specimen Collection Kits
ORDER SPECIMEN COLLECTION KITS
Click here to Order Specimen Collection Kits
- Under the "Kit Information" section of the form, insert:
- Type of kit: Virus Isolation and Detection Kit
- Comments: COVID-19 aka SARS-nCoV-2
KIT CONTENTS
- 1 biohazard bag
- 1 synthetic-tipped swab
- 1 viral transport medium tube
- 1 absorbent pad
Please note: Kit components may vary. Follow storage temperature instructions indicated on the kit. Use only the materials provided with the kits (for example, do not substitute swabs).
PRODUCT STATEMENTS
SWABS: Collection kits may include either nasopharyngeal or nares swabs depending on availability.
VTM: The type of Viral Transport Media in the collection kit may vary depending on availability. Some types of media require refrigeration at 4° C until used and will be labeled accordingly.
QUIDEL SOFIA SARS ASSAY: Per the kit insert "To obtain accurate results, use only Copan UTM or CDC’s formulation of VTM." If your facility uses this assay and requests viral transport media from SHL, please ask for UTM (Copan or BD) or the CDC’s VTM formulation (SHL).
SHL VTM Non-Hazardous Product Statement
The State Hygienic Laboratory is working hard to ensure providers have the materials needed to collect specimens. Please help us conserve resources by only ordering the number of kits immediately needed and reordering as necessary.
Specimen Types and Requirements
PRIOR TO COLLECTION
- All testing for SARS-CoV-2 should be conducted in consultation with a healthcare provider. Specimens should be collected as soon as possible once a decision has been made to pursue COVID-19 testing, regardless of the time of symptom onset.
- Store specimens at 2-8°C for up to 72 hours after collection.
- Label all specimens with name and date of birth. Ensure the information on the specimen matches the test request form. Unlabeled specimens will be rejected and not tested.
COLLECTION PROCEDURES
- Collection instructions for upper and lower respiratory tract specimens are available on the CDC website.
SPECIMEN TYPES
Preferred Specimen:
- A nasopharyngeal (NP) swab placed into viral transport medium (VTM) is the preferred choice for swab-based SARS-CoV-2 testing. To date, the scientific literature states that nasopharyngeal specimens have the highest yield of recovery of an infected patient.
Acceptable Specimen Alternatives:
Upper respiratory
- Oropharyngeal (OP) specimen
- Anterior nares specimen (nasal swab) using a round foam swab
- Mid-turbinate swab
- Nasopharyngeal wash (collect 2-3 mL into a sterile, leak-proof, screw cap container)
Lower respiratory
Collect 2-3 mL into a sterile, leak-proof, screw-cap container.
- Sputum specimens are acceptable for patients with a productive cough. Do not induce sputum collection.
- Bronchoalveolar lavage
- Bronchial wash
- Endotracheal aspirate
Acceptable Swabs:
- Synthetic tips (e.g. polyester or dacron) with a flexible plastic shaft
- Eswab™
- Flocked swabs
Acceptable Transport Medium for Swab Specimens (in order of preference):
The screw-top transport tube should contain a minimum of 2 mL of either:
- Viral transport medium
- Sterile saline or phosphate buffered saline (PBS)
- Liquid Amies transport medium
Multiple specimens may be taken with a single swab. If you use separate swabs from different locations, combine them into a single tube of viral transport media to help preserve testing materials.
- Swabs submitted in bacterial transport media (usually clear fluid)
- Calcium alginate swabs, cotton swab
- Swabs with wooden or aluminum shaft
Specimen Collection and Packaging
Diagnostic PCR Samples using VTM or Saline Transport Medium
Specimen Packaging Guidance (PDF)
Hologic® Panther
SPECIMEN PACKAGING GUIDANCE (PDF)
CAUTION - USE ONLY THE MATERIALS PROVIDED IN THE KITS. REPLACEMENT OF MATERIALS MAY DAMAGE INSTRUMENTATION OR INVALIDATE TESTING.
Important safety notice
In accordance with biosafety requirements, make certain that the screw cap tightly seals the tube. Leaking samples will be rejected.
Specimen Rejection Criteria
Samples meeting any of the following criteria will be rejected and not tested. Facilities will be alerted of a sample rejection via the SHL web portal (for any facilities enrolled in online reporting) or via US Mail.
- Broken or leaking sample containers
- Unlabeled sample containers
- Samples containing swabs other than those provided in the collection kit
- Incomplete or incorrect test request form (must be the Coronavirus COVID-19 Test Request Form)
Result Types and Resources
Result types:
- Detected/Positive - 2019-nCoV COVID-19 RNA was found
- Not detected/Negative - no COVID-19 RNA was found
- Inconclusive - 1 of the 2 targets was detected. Submission of a new specimen is recommended
- Invalid - a result cannot be provided, generally because the specimen was poor quality
| Platform | Analyte Name | LOINC |
|---|---|---|
| Thermofisher TaqPath COVID-19 real-time RT-PCR | 2019 Novel Coronavirus RNA | coronavirus, pcr real-time, 94309-2 |
| Hologic Aptima SARS-CoV-2 | SARS-CoV-2 RNA | coronavirus, tma-cov-2, 94559-2 |
Additional Resources
Running other in-house tests with COVID-19 specimen
It is possible to use the same COVID-19 specimen for other in-house testing. Here's how:
- After swabbing the nasopharyngeal, place the swab in the media, cap, vortex and briefly spin down to remove liquid from the inside of the cap.
- Remove the minimum volume needed to do testing with a transfer pipette, being careful to not splash or drip on the outside of the tube, then re-cap.
- Specimens sent to SHL must have 1 - 2 mL of viral transport media. We prefer to have the swab in the tube, but it is not required.
Serologic Antibody Testing
Testing Criteria and Guidance
Serology Testing Criteria
Serology tests may only be ordered by a healthcare provider.
On February 9, 2021, the Iowa Department of Public Health eliminated testing criteria for SARS-CoV-2 at the State Hygienic Laboratory. SHL will accept any serology test for COVID-19 infection ordered by a healthcare provider with no restrictions.
Use of Serology Testing
While the RT-PCR molecular test is the test to use for diagnosis of COVID-19, detection of antibodies can serve as an indirect marker of infection. For example, if a physician is considering that there may have been a false negative PCR test, serology might be useful for a patient who has tested negative by the molecular assay, is at least 7 days post onset of symptoms and is not immunocompromised. Serology is useful for screening of recovered COVID-19 patients for donors to convalescent plasma therapy. As a surveillance tool, serologic testing is helpful in estimating the prevalence of past viral infection or the cumulative incidence of infection in a population. It can also help improve our understanding of disease transmission patterns and the proportion of people previously infected, among various populations.
It is important to note that there are still many unanswered questions about when to use COVID-19 antibody testing including how detection of antibodies correlates to functional immunity and how long that immunity might last.
Serology Testing for Vaccinated Individuals
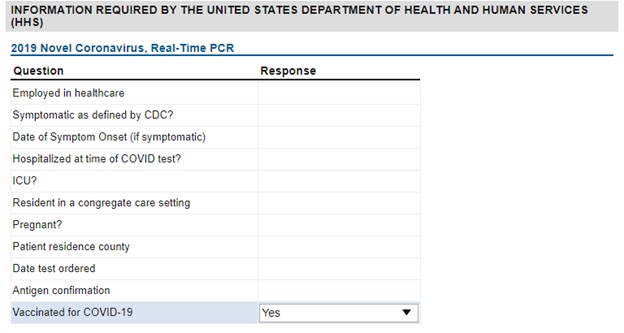
If requesting serology testing for recently vaccinated individuals, please ensure that the HHS supplemental question “Vaccinated for COVID-19” is marked Yes. The COVID-19 vaccines are designed to target the “spike protein” which is found on the surface of the virus that causes COVID-19 disease. By noting that an individual has been vaccinated, the laboratory will be able to order and perform the proper test to determine whether the individual has developed protective immunity against COVID-19.
Test Performance Characteristics
The State Hygienic Laboratory has validated three serology assays with emergency use authorization from the FDA:
- Abbott Architect SARS-CoV-2 IgG
- DiaSorin LIAISON SARS-CoV-2 S1/S2 IgG
- Beckman DxI600 SARS-CoV-2 IgM/IgG
Whichever assay is used, the manufacturer’s reference range is included in the final report.
https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance
Specimen Collection Kits
Serology collection materials are available commercially and should be acquired directly whenever possible.
Materials required:
- Gold or marble top serum separator tubes (SST)
- Plastic biohazard specimen bag
- Blood draw supplies
SHL currently has a limited supply of SSTs available for order. Please help us conserve these scarce resources by only ordering the number of kits immediately needed and reorder as necessary. Please note that SHL kits do not include any blood draw supplies.
Order Specimen Collection Kits
- Under the "Kit Information" section of the form, insert:
- Type of kit: Serology Kit
- Comments: COVID-19
Specimen Types and Requirements
PRIOR TO COLLECTION
SARS-CoV-2 antibody testing should be conducted in consultation with a healthcare provider.
Store specimens at 2-8°C after collection. Specimens may be refrigerated up to 7 days. If storing for more than 7 days, samples must be frozen.
Label all specimens with name and date of birth. Ensure the information on the specimen matches the test request form. Unlabeled specimens will be rejected and not tested.
SPECIMEN TYPES
Preferred Specimen:
1. Serum
Gold or Marble Top Serum Separator Tube (SST) (clot activator w/gel)
1 mL minimum
Prefer to have the specimen centrifuged, but not required. Do not need to aliquot.
Alternative Specimens:
2. Plasma
Green Top Lithium Heparin Tube or Purple Top K2 EDTA Tube
1 mL minimum
3. Serum
Red Top Tube
1 mL minimum
Prefer to have the specimen centrifuged, but not required. Must aliquot and
send serum in a separate tube.
Specimen Collection and Packaging
Serology Samples
Serology Specimen Packaging Guidance (PDF)
Important safety notice
In accordance with biosafety requirements, make certain that the screw cap tightly seals the tube. Leaking samples will be rejected.
Specimen Rejection Criteria
Samples meeting any of the following criteria will be rejected and not tested. Facilities will be alerted of a sample rejection via the SHL web portal (for any facilities enrolled in online reporting) or via US Mail.
- Broken or leaking sample containers
- Unlabeled sample containers
- Incomplete or incorrect test request form (must be the Coronavirus COVID-19 Test Request Form)
Result Types and Resources
Result Types:
Current methods being used are qualitative, with the interpretation of the result being most important. Interpretations are being reported according to the manufacturer’s instructions for the product used. Results will be Positive/Reactive, Negative/Non Reactive, or Equivocal, with Cut-Off Values depending on the method used listed in the Notes Section of the final report.
| Platform | Analyte Name | OpenELIS Test, Method | OpenELIS Report Description | Test LOINC/PHLIP |
Analyte LOINC/PHLIP |
|---|---|---|---|---|---|
| Beckman DxI600 | CoV-2 IgM Antibody | cov-2 igm, cmia-beckman-igm | SARS-CoV-2 IgM Antibody | 94564-2 | 94564-2 |
| Beckman DxI600 | CoV-2 IgG Antibody | cov-2 igg, cmia-beckman-igg | SARS-CoV-2 IgG Antibody | 94563-4 | 94563-4 |
| Abbott Architect | CoV-2 IgG Antibody | cov-2 igg, cmia-abbott-igg | SARS-CoV-2 IgG Antibody | PLT2338 | 94563-4 |
| DiaSorin Liaison | CoV-2 IgG arbitrary units (AU/mL) Result CoV-2 IgG arbitrary units (AU/mL) Interpretation |
cov-2 igg, clia-diasorin-igg | SARS-CoV-2 S1/S2 IgG Antibody | PLT2338 | 94505-5 94563-4 |
Other Resources:
Beckman Coulter
Information for Healthcare Providers
Information for Patients
Abbott Architect
Information for Healthcare Providers
Information for Patients
DiaSorin Liaison
Information for Healthcare Providers
Information for Patients
Whole Genome Sequencing
Whole Genome Sequencing
Update 3/14/2022
The state has lifted the temporary reduction in sequencing sample submission. Facilities should resume submission of their first 10 samples each week to the State Hygienic Laboratory for surveillance of SARS-CoV-2 variants.
Update 09/01/2021
The Iowa Department of Public Health has authorized a temporary reduction in the submission of sequencing samples for clinical facilities experiencing case surges. Facilities should submit the first 5 positive specimens per week, at minimum. As case volumes become more manageable for clinical facilities, the state will return to mandatory submission of all positive samples for sequencing.
--------------------
Order Sequencing
To order sequencing, please use SHL’s “Coronavirus (COVID-19) Sequencing" Test Request Form which is found on SHL’s website. Submit positive samples with a Ct value less than 28. If the Ct value is unknown, submit the sample and SHL will determine the Ct value before performing the sequencing.
Whole Genome Sequencing (WGS) in Iowa
Positive SARS-C0V-2 samples in Iowa should be submitted to the State Hygienic Laboratory for sequencing. Not all samples can be successfully sequenced as it is dependent on the concentration of virus in the individual’s specimen.
Sequencing results are for surveillance and public health epidemiology purposes only. Results will not be reported to submitting facilities or to the individual patient.
Sequencing will provide IDPH with much more information about the variants that are circulating in Iowa now and into the future. Declining utilization of testing makes it even more important to perform sequencing on all positive samples to determine if new variants are emerging. Submitting samples to sequence depends on the cooperation of all laboratories in Iowa.
Requesting WGS
If your laboratory has remaining samples that have tested positive for SARS-CoV-2 and the Ct value is less than 28, please submit the sample to SHL. If the Ct value is unknown, then submit the sample and SHL will determine the Ct value before performing the sequencing.
To order sequencing, go to SHL’s website and select the “Coronavirus (COVID-19) Sequencing" Test Request Form.
Patients must work with their healthcare provider.
WGS Results
SHL is only authorized to report sequencing results to the Iowa Department of Public Health for public health surveillance purposes. Sequencing is not a CLIA-approved test. Healthcare providers should call IDPH at 800-362-2736 for further questions.
What is WGS?
Whole genome sequencing determines the order of bases in the genome (genetic material) of an organism. A genome consists of multiple genes which determine the characteristics of the organism.
WGS in the COVID-19 Pandemic
Genome sequencing enabled the world to rapidly identify SARS-CoV-2 and later develop diagnostic tests and other tools for the response. Continued COVID genome sequencing will be important for public health officials to track the transmission routes of the virus globally, detect mutations quickly to prevent the spread of new strain types, identify viral mutations that can avoid detection by established molecular diagnostic assays and affect vaccine potency, and screen targets for possible COVID-19 therapeutics.
The Importance of WGS
There is growing concern over fast spreading, novel variants of the SARS-CoV-2 coronavirus, such as the B.1.1.7 strain originally identified in the United Kingdom. This novel variant is more easily transmissible than other SARS-CoV-2 virus strains and infection with this strain may lead to approximately 30% higher mortality rates.
More Information
For more information see https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/variant-surveillance.html
Other
BinaxNOW Point of Care Antigen Testing
Supply Update: March 22, 2022
The state of Iowa's supply of CLIA-waived Abbott BinaxNOW Ag tests, distributed by SHL, is out of stock. The manufacturer is unable to fulfill Iowa's order and no additional product is expected in the future.
EUA
The FDA has issued an EUA for Abbott BinaxNOW COVID-19 Ag Cards.
NOTICE: The FDA has alerted clinical laboratories of the potential for false positive results with antigen tests. Read more here.
Extension of BinaxNOW Expiration Date
Abbott issued an updated Product Expiry Extension for BinaxNOW COVID-19 Ag Cards in August 2022. This announcement lists all lot numbers with FDA-approved product expiry date extensions.
Additional details are available on the Abbott BINAXNOW™ COVID-19 AG CARD Training site.
Specimen Delivery
Courier:
SHL utilizes Central Delivery Service of Iowa for courier service. If you are a current CDS client, go to the CDS webpage to arrange a pick-up. If you are not a current CDS client, call SHL at 1-855-374-4692 for assistance.
Please help expedite sample pick-ups:
- Samples must be prepared in advance of pick-up. The CDS driver is authorized to leave the site if asked to wait for sample preparation.
- If your facility is locked-down, please make every effort to get the driver so they are not delayed by screening lines.
Clearly label the outer most package with:

- To: Name of Testing Lab (e.g. SHL)
- From: Your facility name
- COVID-19
Hand-delivery:
Bring to the State Hygienic Laboratory Sample Drop-Off window in Coralville, IA during business hours. A drop box is available at the Coralville lab Sample Drop-Off door for bagged specimens after hours.
Shipping:
Ship overnight via UPS or FedEx following Category B shipping requirements. For specific details about shipping “Biological Substances, Category B” through UPS or FedEx, or to order packaging and labeling materials, please contact the respective agency directly.
-
FedEx
-
FedEx webpage on “How to Ship Dangerous Goods”
-
FedEx Dangerous Goods/Hazardous Materials Hotline: 1-800-463-3339 (say “dangerous goods”)
-
-
UPS
-
UPS Webpage on “Infectious substances”
-
UPS Hazardous Materials Support Center: 1-800-554-9964
-
Laboratory Risk Assessments
Laboratory Biosafety Guidance: Visit https://www.cdc.gov/coronavirus/2019-ncov/lab/index.html for the most current CDC guidance.
Each laboratory in Iowa can prepare to receive samples for COVID-19 by doing the following:
- Perform a risk assessment for receiving and handling specimens from patients under investigation for COVID-19
- Use appropriate personal protective equipment (PPE)
- Ensure biological safety cabinet (BSC) certification is current
- Train all applicable staff on the proper use of a BSC. CDC Fundamentals of Working Safely in a Biological Safety Cabinet
- Refresh staff knowledge regarding Category B Packaging and Shipping
- Make certain that all staff follow the established disinfection protocols
- Remind staff of the importance of proper and regular handwashing
- Train staff on the signs and symptoms of COVID-19
- Contact the State Hygienic Laboratory with any concerns